Thermal conductivity and melting point of aluminum properties
Aluminum has excellent mechanical properties, making it ideal for a variety of applications. One of the qualities that sets it apart from other materials is its thermal conductivity. Among commonly used metals, copper and aluminum both have excellent thermal conductivity, making aluminum a good choice for tasks involving regulating or transferring heat.
While certain aspects of aluminum tend to get all the attention, such as its high strength-to-weight ratio, excellent corrosion resistance and good formability, thermal conductivity is often overlooked. Aluminum conducts heat far better than stainless steel and other metals, making it the choice of manufacturers in many industries, including electronics, plastics and aerospace.
A question we are often asked is how hot aluminum can get before it becomes a problem. People wonder how much heat can be applied to aluminum parts and machinery before the material fails. These questions all boil down to two main principles: thermal conductivity and melting point; and that's what we're going to discuss today.
How is thermal conductivity measured?
When we talk about the thermal conductivity of a material, we mean its ability to conduct heat. In scientific terms, this is specified as a number based on the so-called Fourier's law, which states that the rate of heat transfer through a material is proportional to the negative gradient of temperature and area, at right angles to this gradient, through which heat flows. This is a complicated way of saying that thermal conductivity tells us how quickly heat is transferred through a material. In general, the higher the number, the faster the heat transfer.
It's also important to note that even for pure aluminum, the actual amount will vary with heat; calculating thermal conductivity can be more complicated for various alloys. You should never assume that the lab number for thermal conductivity is correct, as you will need to test your application in various scenarios to ensure how it handles various temperatures.
Let's look at some real world examples. Styrofoam, commonly used as insulation, has very poor thermal conductivity. Styrofoam cups are great for hot coffee because it doesn't transfer the heat of the liquid to your hand holding the cup. Metals like aluminum, on the other hand, have excellent thermal conductivity. This means that if you have an aluminum mug filled with extremely hot coffee, the mug itself will be hot to the touch and difficult to hold.
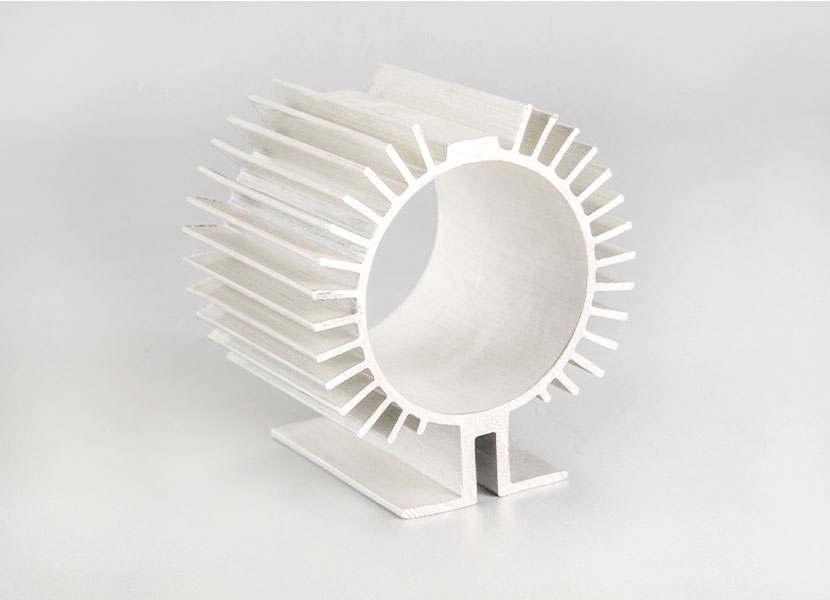
A radiator is a passive heat exchanger in which heat generated by an electronic or mechanical device is transferred to air or liquid coolant, thereby preventing the device from overheating. Common uses for heatsinks are CPUs and GPUs, which tend to get very hot and can be damaged by overheating. Aluminum is common in such devices due to its thermal conductivity and light weight.
Another industrial application that benefits from aluminum's high thermal conductivity is plastics processing. When molten plastic is solidified into a finished product through an injection molding or blow molding process, the solidification time in the mold depends on the thermal conductivity of its material. Using aluminum instead of steel reduces cycle time for manufacturing parts, increases productivity and reduces valuable stamping/machine time.
melting point of aluminum
Of course, this thermal conductivity is good only to a certain extent. If the metal is heated too much, it will start to deform, so you must know the material's melting point and how much heat it needs to withstand before using it in your application. In other cases, knowing the melting point of aluminum is critical, such as when welding or heat treating aluminum alloys.
What is the melting point of aluminum? If you looked it up in a textbook, the answer would be 1,221°F (660.3°C), but manufacturers almost never use pure aluminum. Each alloy has a different melting point, and some alloys are specially made for use in high temperature environments. There are high-strength aluminum alloys alloyed with Zn, Mg, Cu, and Sc, which have melting points as high as 1275°F.
Another major concern with high temperature applications of aluminum is that the mechanical properties of the metal will be affected. Higher alloy grades strengthened by heat treatment processes lose these higher mechanical properties at high temperatures. Exposure to excessive heat will temper and weaken heat-treated metals.
If your application will experience high temperatures, it is important to test it carefully during the prototype stage, especially if durability is an important consideration. Choosing the right alloy that will perform well under specific conditions is key to securing your bottom line. That's why working with an experienced material supplier can help you save time and money.

 English
English 简体中文
简体中文 España
España